Welcome to AIM-HI Lab
Advancing care through artificial intelligence and medical imaging
About Our Lab
The AIM-HI Lab at Cleveland Clinic's Department of Urology is dedicated to developing and applying artificial intelligence solutions to improve the lives of those affected by urologic diseases. We use advanced computing approaches to enhance diagnosis, treatment planning, and patient outcomes. Our research focuses on three key areas: the use of computer vision to maximally extract data from medical images, leveraging large language models for efficient extraction of clinical data helping every patient to become an information donor and drug discovery. By combining cutting-edge AI technologies with clinical and translational research expertise, we aim to enhance the precision and efficiency of urologic care while maintaining the highest standards of patient safety and data privacy.
Lab Leadership
Christopher Weight, MD
Clinical Director-Cancer Lead
Jack Weaver, MD
Pediatric Lead

Glenn Werneburg, MD
Drug Discovery Lead
Jihad Kaouk, MD
Chairman of Dept of Urology
Our Research
Recent Publications
AI Age Discrepancy: A Novel Parameter for Frailty Assessment in Kidney Tumor Patients
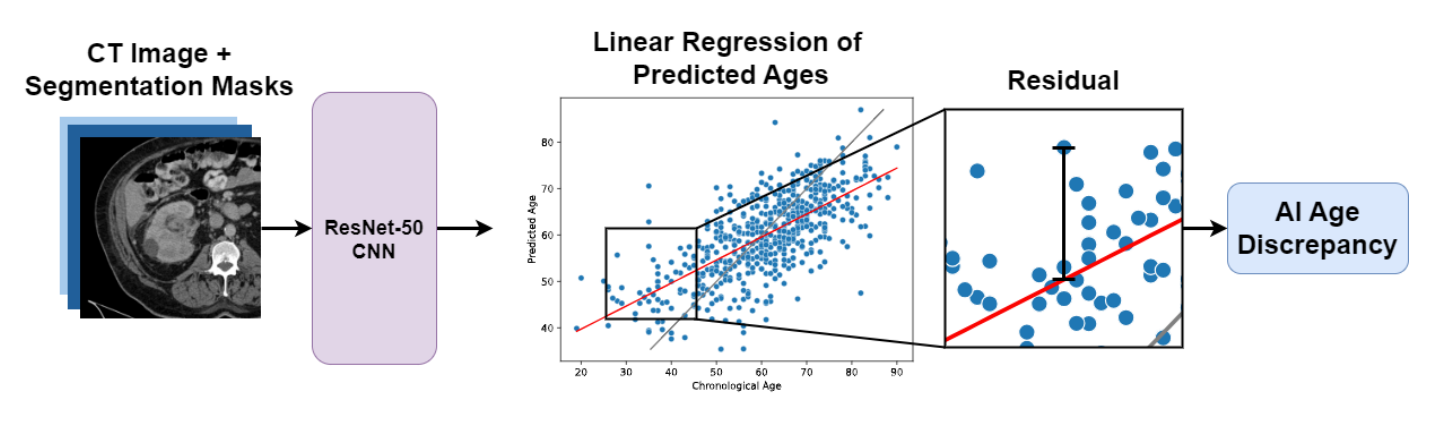
arXiv preprint arXiv:2407.00438 (2024)
This paper introduces AI Age Discrepancy, a novel metric derived from machine learning analysis of preoperative abdominal CT scans, as a potential indicator of frailty and postoperative risk in kidney cancer patients. This retrospective study of 599 patients from the 2023 Kidney Tumor Segmentation (KiTS) challenge dataset found that a higher AI Age Discrepancy is significantly associated with longer hospital stays and lower overall survival rates, independent of established factors.
The KiTS21 Challenge: Automatic segmentation of kidneys, renal tumors, and renal cysts in corticomedullary-phase CT
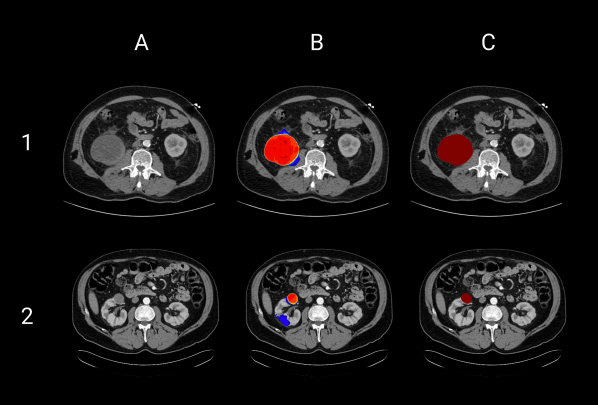
arXiv preprint arXiv:2307.01984 (2023)
This paper presents the challenge report for the 2021 Kidney and Kidney Tumor Segmentation Challenge (KiTS21) held in conjunction with the 2021 international conference on Medical Image Computing and Computer Assisted Interventions (MICCAI). KiTS21 is a sequel to its first edition in 2019, featuring innovations in challenge design and a larger dataset. The challenge used a novel annotation method with three separate annotations for each region of interest, performed in a fully transparent setting using a web-based annotation tool. The test set was collected from an outside institution, challenging participants to develop methods that generalize well to new populations. The top-performing teams achieved significant improvements over the 2019 state of the art, approaching human-level performance.
Computer Generated R.E.N.A.L. Nephrometry Scores Yield Comparable Predictive Results to that of Human-Expert Scores in Predicting Oncologic and Perioperative Outcomes
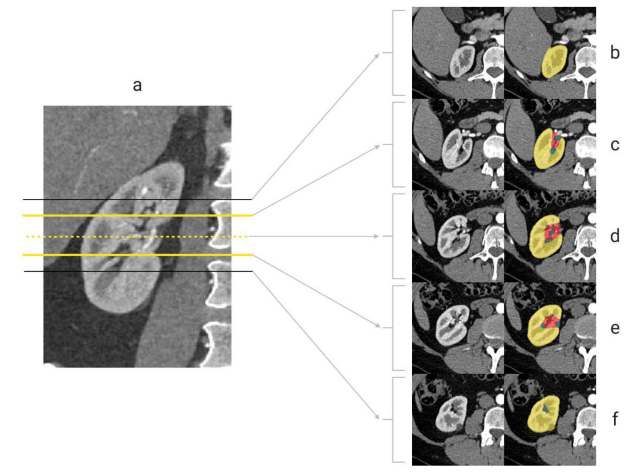
The Journal of Urology, Volume 207, Issue 5, May 2022 (pp. 1105–1115)
Leveraging deep learning for semantic segmentation of kidney CT scans (n = 300), this work automatically computes R.E.N.A.L. nephrometry scores ("AI-Scores") and compares them to human-expert ("H-Scores"). AI-Scores showed substantial concordance with H-Scores (Lin's ρ = 0.59) and proved equally predictive of malignancy, high tumor grade/stage, and key perioperative metrics (e.g., surgical approach, estimated blood loss). By removing manual scoring variability and saving clinician time, the AI-based system aims to facilitate broader adoption of standardized nephrometry in renal tumor care.
Public Perceptions of Artificial Intelligence and Robotics in Medicine
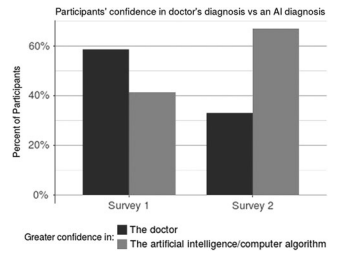
Journal of Endourology, Volume 34, Number 10, October 2020 (pp. 1041–1048)
This survey-based study (n = 264) assessed how laypersons perceive AI and robotic surgery in oncologic care. Although most participants expressed similar trust in physician vs. AI‐based diagnoses, they were significantly more likely to trust AI for cancer detection when primed to believe AI can diagnose. Additionally, 88% mistakenly thought partial autonomy in robotic surgery already exists, and over half reported discomfort with robotic procedures—highlighting the need for clear patient counseling as AI/robotic tools become more prevalent.
Contact Us
Department of Urology
Cleveland Clinic
9500 Euclid Avenue
Cleveland, Ohio 44195
Email: hellern@ccf.org

